Virtual technology may replace traditional therapy for those recovering from surgery and those with lung conditions, such as the overproduction of mucus, with a recent study showing the benefits of a new browser-based smartphone app designed to deliver such therapies.
Developed by Queensland University of Technology (QUT) PhD candidate Clarence Baxter, the browser-based ‘QUT Inspire ISy’ app has been tested and compared with the TriFlo II clinical incentive spirometry device in a recent study involving 24 healthy participants, with the results published in the BMJ Open Respiratory Research journal.
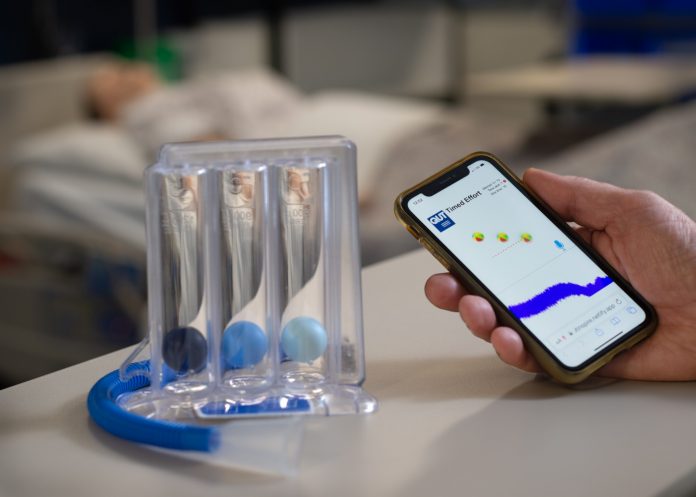
The QUT ISy app is a web app using the built-in microphone available in all smartphones to detect inspiratory sounds. This offers a responsive animated graphic display for as long as detectable breath sound is sustained.
Mr Baxter says the app is a “virtual” form of a longstanding clinical incentive spirometer (ISy) used to detect a person’s inspiratory breath, intended to encourage persistence with repeated gradual maximal inspirations for respiratory therapy.
“The app was developed as a low-cost, easy-to-use way to promote repeated deep inhalations by patients to dislodge mucus from the airways so that it can be expelled by coughing,” he said.
“Removing mucus from the lungs is important when recovering after surgery, as it may collect in the tiny air sacs, or alveoli, of the lungs while a patient is prone for a long time, presenting risks of developing respiratory infections or pneumonia.
“The traditional clinical ISy device has a tube from which users inhale in order to create a vacuum to raise three spheres in separate clear chambers. The aim is to keep the three spheres aloft using a sustained slow deep inhalation to encourage dislodgement of mucus from the airways.
“Obviously, the user can see how they’re doing by watching the spheres rise in the clinical ISy device, and so we used this visualisation virtually in the app to give users the same visual cues regarding their inhalations.”
In the first phase of this new clinical study, participants compared three gradual maximal inhalations using the QUT ISy app with three inhalations using the clinical ISy device.
“We randomly assigned the testing order so that some participants used the app first and the ISy second, and vice versa, to avoid any device testing order effects,” Mr Baxter said.
“Usability was measured for three factors: effectiveness – the duration of sustained inhalation effort; efficiency – assessed by using the distance participants held their phones from their mouths; and user satisfaction, assessed by a post-test questionnaire and qualitatively through semi-structured interviews.
“No significant difference was found between the duration of inhalations (in seconds) using the QUT ISy app compared with the TriFlo II clinical ISy device,” he said.
Mr Baxter says that while participants were asked to hold the phone less than 5cm from their mouths to capture the inhalation sound, most held their phones on average about 13cm away, possibly to better view the screen.
“Even so, this distance was within the detection range of approximately 50cm for medium and high inhalation flow sounds identified in an earlier simulation study using this app,” he said.
“User satisfaction was high, with most participants preferring the new virtual app as best for displaying inspirations. They reported the app’s screen display had the advantage of responsiveness and compactness over the TriFlo II.”
Mr Baxter says people with cystic fibrosis have ongoing overproduction of mucus (including mucus in the airways) and could benefit from using the app, as they would need no other equipment other than their smartphone.
“Other potential users include people with chronic obstructive pulmonary disease, who may have reduced lung elasticity or other pathological lung changes resulting in accumulation of mucus,” he said.
“As this study shows, healthy participants reported the app encouraged inhalations of comparable vigour and duration with the traditional IS device, further studies with more vulnerable groups with these relevant disease conditions are warranted.”
For more information, visit: bmjopenrespres.bmj.com/content/9/1/e001221
This article was originally published in the September issue of Retail Pharmacy magazine.