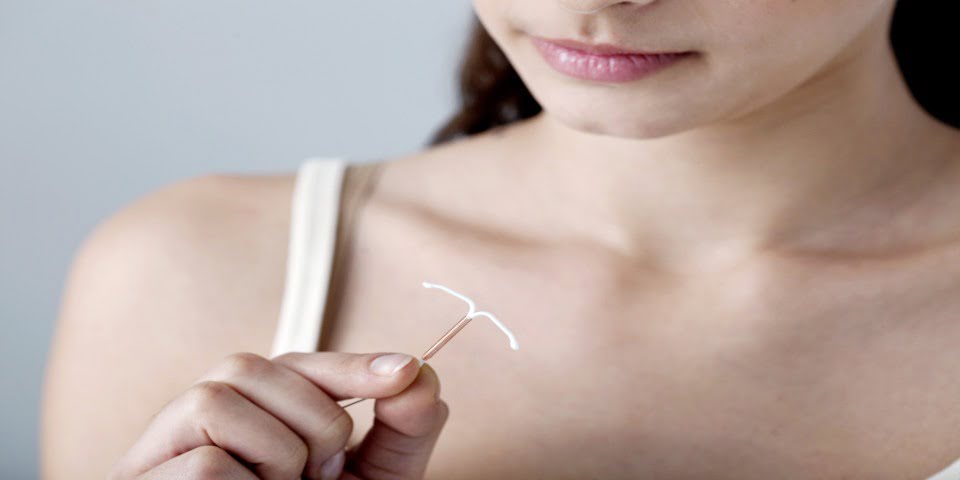
Law firm Slater and Gordon will pursue a class action against Bayer, the manufacturer of contraceptive device Essure, after hundreds of women across Australia suffered severe complications.
Essure, recalled by Bayer in the US in July, is a permanent contraceptive implant comprising a metal coil that expands to anchor the device in the fallopian tube.
“The Essure device has been known to corrode, exposing women to nickel poisoning, as well as migration and perforation of the uterus and other organs,” Slater and Gordon said..
The device is also said to cause complications including irregular menstrual bleeding, pelvic or abdominal inflammation and pain, pain during intercourse, reduced libido, stiffness and muscle pain, and symptoms including fatigue, hair loss and rashes. Expert medical opinion has suggested a complete hysterectomy is the only safe way to remove the product.
Slater and Gordon Associate Ebony Birchall says the firm will consider whether the product was inherently defective.
“Essure was hailed as the new wave of contraceptive devices,” she said. “Unlike traditional permanent contraceptive surgery, Essure was marketed as being fast, effective and minimally invasive. It could be inserted in your doctor’s office.
“However, for the women who have experienced complications, it has been incredibly damaging. It has turned their lives upside down.
“For most women, the only solution has been to have a complete hysterectomy.”
Ms Birchall says a large number of women with serious complications from the Essure implant have contacted Slater and Gordon, and there are probably many more who are suffering in silence or who do not know their symptoms are related to the device.
Tanya Davidson, whose husband was a fly-in, fly-out worker, had the device inserted in 2010 and says she has battled severe effects for the past eight years. She has experienced symptoms such as hair loss, severe menstrual bleeding, chronic fatigue, gastric issues, stabbing ovarian pain and loss of cognitive function.
“The loss of cognitive function has been the scariest, as it just feels like it continues to get worse,” she said.
“Every day I wake to the feeling of brain fog, have trouble remembering simple things like the names of everyday objects, or get lost mid-sentence.
“I was terrified I was experiencing the onset of early Alzheimer’s disease.”
Ms Davidson says that when she first decided to have the device implanted it seemed like the best option.
“As a busy working mum, I couldn’t afford to take time off, so the fact that the procedure was non-invasive and I didn’t have to take time off to recover from surgery was appealing,” she said. “Instead it has been eight years of hell.”
Ms Davidson was eventually diagnosed with a nickel allergy, and in February 2016 had the device removed.
However, during that procedure the device broke and she had to undergo a hysterectomy six months later as a result of the damage caused by the remaining fragments. She still suffers from side effects.
“Some days I just feel like I can’t get out of bed, but as a mum I don’t have a choice,” she said.
“For years doctors told me that the symptoms were in my head and that they couldn’t be related to the device.
“I know there must be other women out there who are in the same boat, and I want them to know they are not alone.”
Bayer has not provided details of how many of the devices were sold in Australia or worldwide.
The class action is open to all women who have suffered complications as a result of having an Essure implant inserted in Australia. For more information, visit https://www.slatergordon.com.au/class-actions/current-class-actions/essure-class-action.
Further, the TGA notes that Essure was cancelled from the Australian Register of Therapeutic Goods on February 9, 2018, but no new Essure devices had been supplied for the Australian market after May 31, 2017. Since 1999, Essure has been the subject of 59 adverse event reports.