The PBS listing of two of 2 biologic medications for severe psoriasis was announced overnight by Federal Health Minister Greg Hunt.
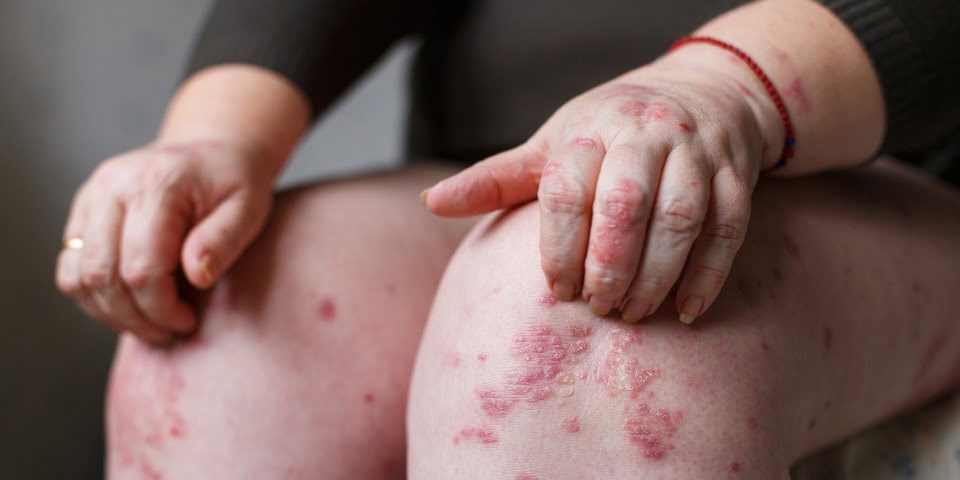
The estimated 19,000 Australian adults living with severe chronic plaque psoriasis will welcome the additions, available on the PBS for those who meet the reimbursement criteria.
From Feb 1st, Ilumya, (tildrakizumab) from Sun pharma and and Tremfya (guselkumab), from Johnson & Johnson are both part of new class of biologics that specifically inhibit the action of interleukin-23 will become available on the PBS.
The listings coincide with an article published in MJA Insight this week, highlighting the substantially unrecognised burden of psoriasis and general lack of awareness among GPs and the broader patient community, of the availability and efficacy of biologic therapies, which may be delaying patient access to effective, long-lasting treatment.
Clinical dermatologist and Director of Research, Skin & Cancer Foundation Inc., Associate Professor Peter Foley says although there is no cure for the immune-mediated disease, the advent of new biologics targeting the IL-23 cytokine represent a promising development for the estimated 19,000 Australians living with severe forms of plaque psoriasis.
“While psoriasis was once thought of as little more than ‘influenza of the skin’, our improved understanding of the immunological pathways involved in the disease has led to the development of biologics with a more targeted mechanism of action,” he said.
“The IL-23 inhibitors represent the latest evolution of targeted biologic agents for severe psoriasis. Trials data has demonstrated their durability of response and they appear to be well-tolerated, with low frequency of serious adverse events.”
The new drugs will be listed from February 1st 2019